Costum built high resolution force spectroscopy combined with imaging
Research for a better understanding of biological processes from a single molecule towards a cellular or even tissue scale is strongly dependent on the avialability of cutting edge instrumentation that often does not exist yet on the market. Therefore, to answer some specific research questions, custom built adaptation of existing instrumentation is needed. During my master and PhD thesis I developed an atomic force microscope that allows the usage of small cantilevers with an increased signal to noise ratio and during my PostDoc studies I implemented an optical trap with a force-feedback loop into a commercially avialable confocal microscope from Nikon for the study of cellular mechanosensing.
High resolution atomic force spectroscopy
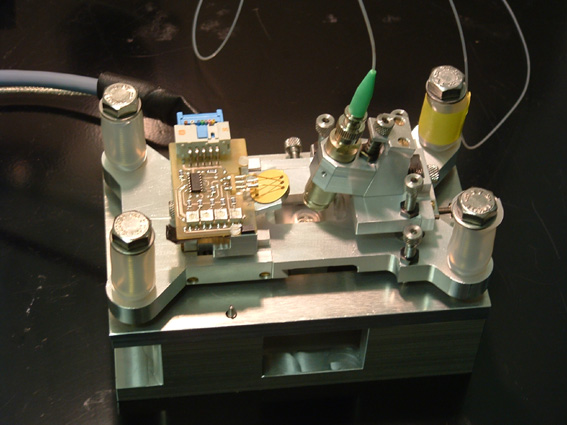
The core of an atomic force microscope (AFM) is a bendable cantilever with an atomically sharp tip that is mounted at its end. The stiffness of the cantilever can be calibrated via the thermal noise spectrum and forces in the pico-Newton regime can be measured via detection of the deflection of the cantilever. This is done by detecting the movement of a laser beam that is reflected from the back of the cantilever and analyzed with a 4 quadrant photodiode. The picture shows the design of an AFM for smaller cantilevers with less thermal noise. Down-sizing of the focal spot asked for an improved design of the optical path especially at the air/plastic/water interface. The instrument is avialable in the Rief-lab and you can find more information on the technical specifications here.

Combining confocal microscopy and optical tweezers
Optical tweezers allow to trap micrometer sized particles in aqueous solution using a focused laser beam. Already small forces in the pico Newton regime will move the bead away from the trap focus and can thus be detected. Optical tweezers can easily be added to light microscopes. The picture shows the optical path of the trapping laser emitting 1070 nm light, that was added to a commercial Nikon Eclipse confocal microscope. Using dichroic mirrors and filters allows to acquire fluorescence images in the visible spectrum while simultaneously controlling and recording the forces acting on the optically trapped particle. The instrument is avialable in the Bassereau-lab and you can find more information on the technical specifications here on page 3.
Imaging approaches for skin and hair research
As an entirely cosmetic company, L'Oréal focusses its research on skin and hair. The skin is our largest organ and defines the barrier that seperates our body from the outside. This barrier function is in many cases essential for our health, for example it prevents harmful raw materials such as pollutants to enter. In other cases e.g. for the delivery of medical drugs it can be advantageous to decrease the skin barrier function locally for proper drug delivery as well as for cosmetic porposes. The quantification of this barrier function for the penetration of different raw materials, the examination of biological processes that lead to the proper structural formation of skin and its maintainance, as well as the biology behind skin aging are among the main tasks we adress in the imaging platform. In addition we want to better understand the biology behind hair growth and the biochemistry of the formed, keratinized hair fiber. To give structural and mechanistic insight in those questions we use different imaging techniques.
Electron microscopy
To study the surface properties of skin and hair e.g. after an cosmetic treatment, we use scanning electron microscopy. The usage of an EDX detector allows in parralel to gain insight in the chemical distribution of different raw materials used in our formulations as well as on the surface of e.g. the hair fiber. Cryo electron microscopy allows to visualize cosmetic formulations in their native state. Using transmission electron microscopy (TEM) we gain ultrastructural insight in skin and hair and e.g. how the hairs ultrastructure is altered due to exposure to different pollutants or UV light. The TEM picture on the right compares the structure of the hair cortex when containing an high concentration of pollutants (upper half) with one containing less pollutants, you can find more details here.

Confocal, multiphoton and fluorescence lifetime microscopy
Light microscopy is due to its non-invasiveness especially well suited for the study of skin. Multiphoton microscopy combined with Fluorescence Lifetime Imaging Microscopy (FLIM) allows to study the morphology of epidermal and upper dermal layers of the skin in-vivo in a non-invasive manner. We use the signal coming form the second harmonic generation (SHG) to specifically detect collagen and the short fluorescence lifetime to detect melanin. This allows us to study structural changes as well as changes on the melanin distribution after product application in-vivo. The background image on the left shows z-stacks when entering the skin (from upper left to lower right), blue shows the autofluorescence of skin and red the collagen signal. You can find a recent overview about this topic here.
Raman spectroscopy
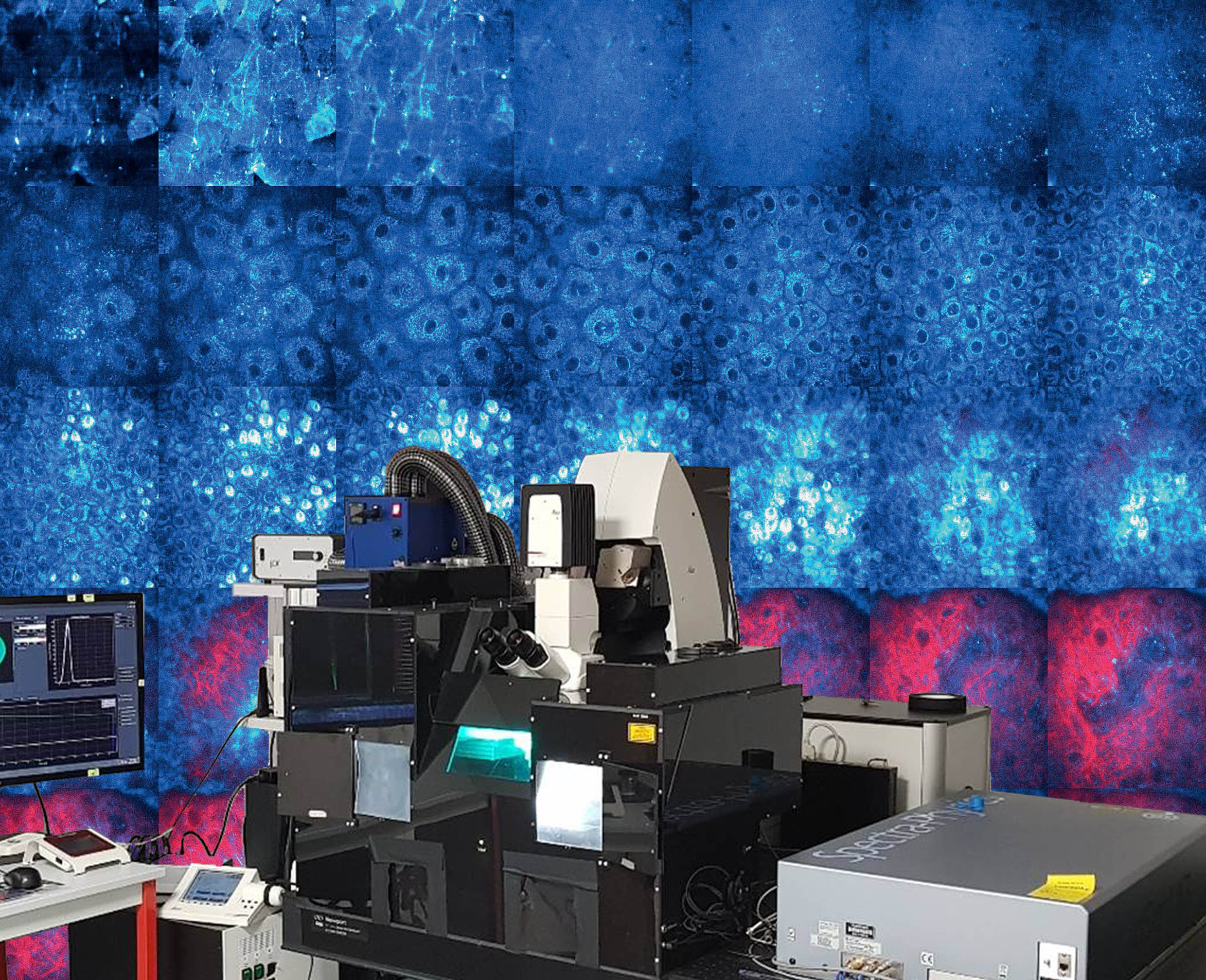
We use Raman spectroscopy to study skin parameters such as the water content or internal lipid organization on reconstructed skin as well as in-vivo. Raman spectroscopy also allows to follow product penetration through the skin. The picture shows a commercial Raman microscope and concentration maps of deuterated glycerol in different skin depths in-vivo that we measured using Coherent Antistokes Raman Spectroscopy. You can find more details here


Atomic force microscopy
Besides its capacity to measure piconewton forces on a molecular lenghtscale, atomic force microscopy also allows to aquire topographic images with high resolution. We use atomic force microscopy to study the mechanics of skin and hair and their microstructure. For example we followed the keratinization process of the hair fiber within the first millimeter of a human hair follicle. The image shows a commercial atomic force microscope and a picture of the macrofibril organization within the hair follicle. You can find more information here