Exposure to pollutants leads to accelerated degradation of human hair
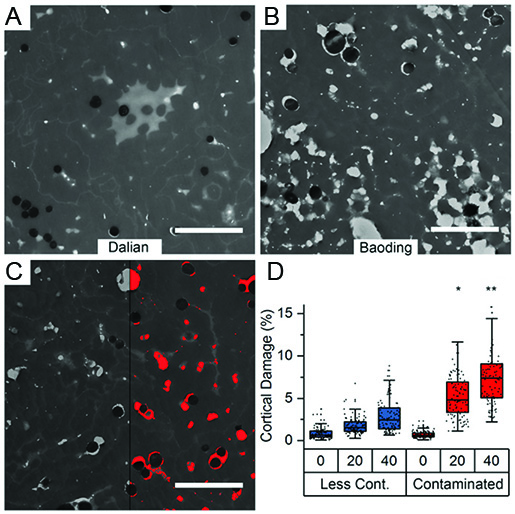
With the rise of industrialisation, air pollution via phototoxic polycyclic aromatic hydrocarbons (PAHs) became a major risk factor for human health. While in-vitro observations and in-vivo correlations suggest a detrimental effect of PAHs at physiological concentrations, in-vivo observations of the structural impact of PAHs on biological tissue are rare. We use transmission electron microscopy on human hair fibers collected from 200 volunteers living in two different cities with different air pollution exposure. A first analysis showed that fibers from the more polluted city (Baoding, figure B) show more internal damage as compared to fibers from the less polluted city (Dalian, figure A). Exact quantification of 25 biomarkers for PAH exposure in all fibers allowed to show an increased structural degradation of the hair fiber over time, when increased PAH concentrations are present (Figure C and D). [read more]
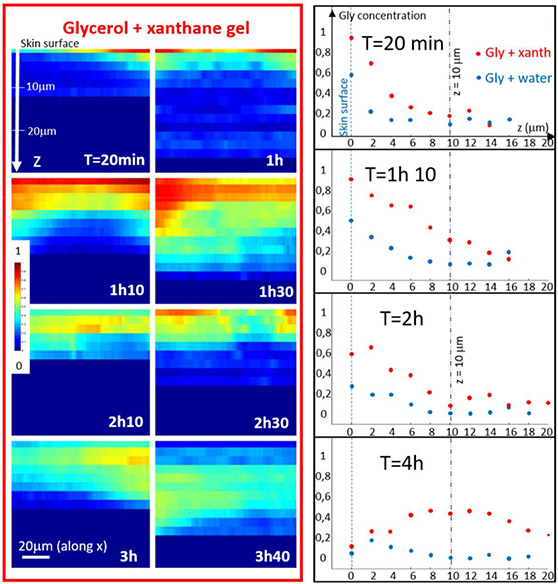
Coherent anti-stokes raman spectroscopy allows to follow glycerol penetration through skin in-vivo
The stratum corneum is the outermost layer of the skin, protecting us from unwanted penetration of environmental compounds. However, for some medical or cosmetic treatments penetration of specific raw materials through the skin is desirable. To quantify the fraction of raw materials, that are overcoming the skin barrier, different invasive [read more] and non-invasive methods [read more] can be used. Raman spectroscopy based methods can allow to specifically follow product penetration through the stratum corneum if the fingerprint spectra of the product is discernable from the skin spectrum. Based on a previous study on ex-vivo skin [read more], we used Coherent anti-Stokes Raman spectroscopy to follow glycerol penetration through the stratum corneum in-vivo. [read more]
Mapping the mechanics of the hair follicle
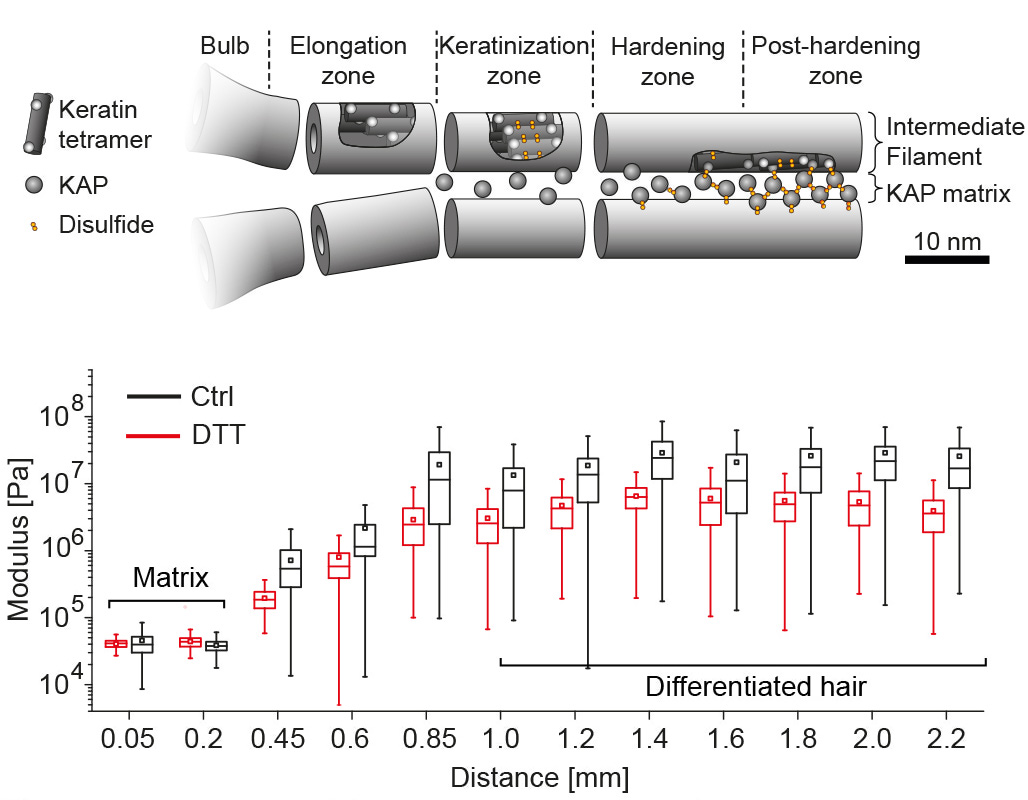
Hair is a mechanically remarkable substrate, dry hair has a stiffness of several GPa which is comparable to the Youngs modulus of different plastics such as nylon. This high elastic modulus is achieved already a few millimeters above the base of the hair follicle, from which the hair protrudes. As the living keratinocytes at the base of the follicle divide, they push older keratinocytes outwards forming over time a dense keratin network via a process called keratinization. We used atomic force microscopy to map the related mechanical stiffening along the hair follicle fiber, observing a 360 fold increase in stiffness of a follicle immersed in aqueous solution over the first millimeter. [read more]
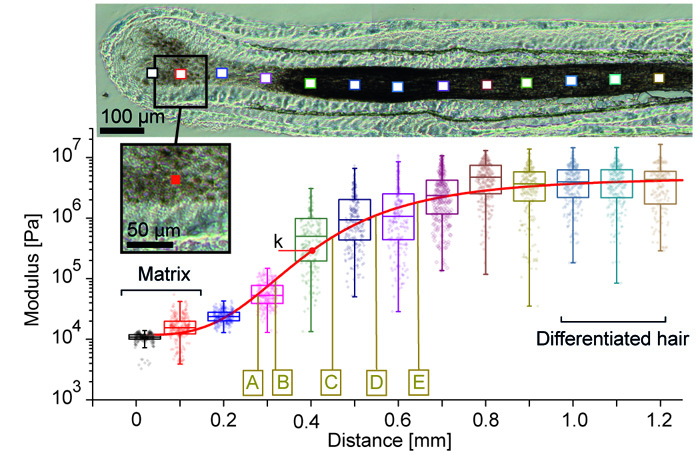
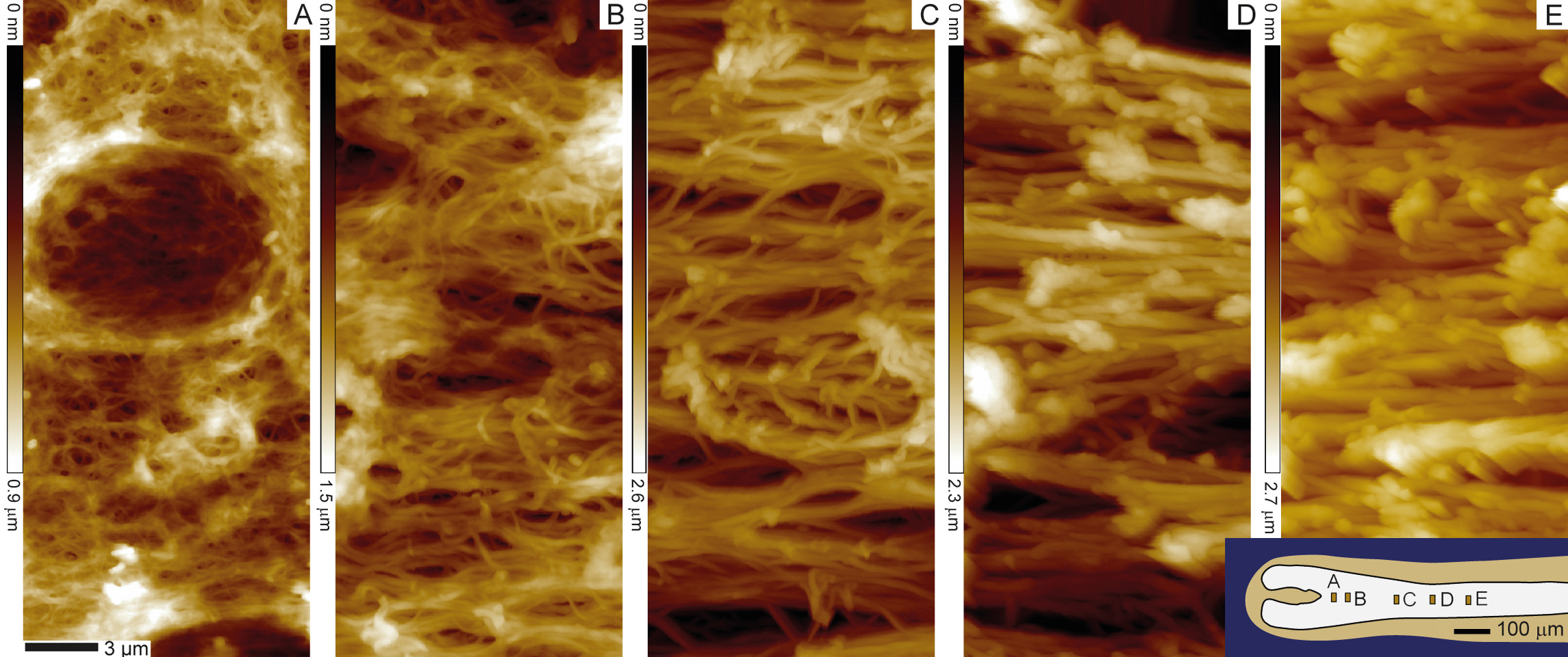
Mapping macroscopic changes in the keratin architecture of the hair follicle
Atomic force microscopy can also quantify the structural changes in the keratin network formation that leads to the observed stiffening. We quantified that over the first 0.5 mm along the follicles hair fiber, keratin fibers thicken while they become more and more aligned and more and more compacted.
Internal reorganizations of the keratin macrofibers
Using x-ray micro-diffraction (XRD) along the hair follicle we can correlate the mechanical stiffening with internal structural changes of the macrofibers. In agreement with earlier publications we found a heterogeneous and amorphous organization of the early intermediate filament network at the base of the follicle, that becomes more organized and compact also via the setting up of inter-molecular cysteine bonds. Using a strong detergent that opens cysteine bonds reduces the stiffness of the fiber in later keratinization stages, confirming the XRD data and the available literature about the keratinization process in the early hair. [read more]