Filopodia are sensory cell antennae
Cells can sense the mechanics of the substrate and hair-like structures called filopodia may be involved in this process. Filopodia are found in different cells such as fibroblasts or neurons where they sense the chemical environment of a cell. For example, when the back of the fruit fly closes during embryogenesis, filopodia create contact with matching partner cells and pull on the opposing cell layer. Axons show filopodia on their growing end where they seem to pull the axon toward a chemical signal. Their dynamics and behavior can be observed in vitro on cells plated on 2D glass slides but not much is known about how they produce force. [read more]

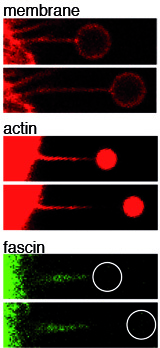
How filopodia pull
Using confocal microscopy together with optical tweezers we can approach a specifically coated bead to the tip of a single filopodium to quantify related forces. This approach showed that filopodia pull via the membrane tension and the internal actin that is constantly pulled backwards by the cell. At high forces the soft filopodial membrane stays attached to the bead, while the rigid actin detaches locally at the tip as shown in the snapshots. This shows that the tip is the weakest point of actin based force transduction and that the tip could pull with defined forces by controlling the amount and strength of the internal molecular tip-links. [read more]
The sense is in the finger tips
The tip of a filopodium contains many actin regulating proteins and often makes first contact with the environment. We showed that the tip quickly responds on extracellular mechano-chemical signals. The tip controls the speed of actin polymerization which depends on substrate adhesion and on the mechanical signal of forced rupture. This allows filopodia to continuously pull on the substrate and thereby probe its surrounding with a load-and-fail behavior. [read more]
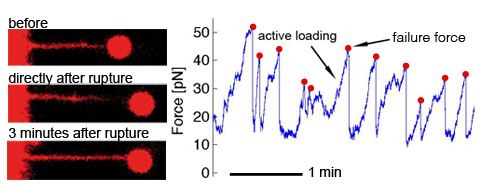
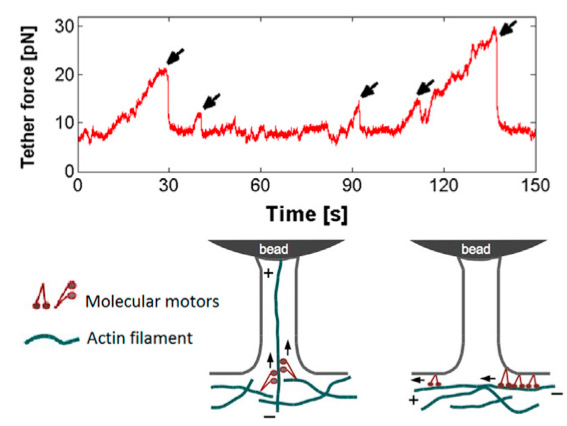
Load and fail behavior of membrane tethers
While active pulling and with sufficient counter forces also active load and fail behavior is exerted by filopodia from different cell types, it is usually not observed when membrane tethers are artificially pulled from cells. However, certain cell types can also show active load and fail behavior of artificially pulled membrane tethers when they are held for longer times, due to actively growing actin inside the initally empty membrane tether. Interestingly, although axons have a cytoskeletal architecture that is very different from e.g. ephitelial cells, they also show active load and fail behavior of pulled membrane tubes indicating a similar underlying mechanism [read more].