Mechanical forces and molecular motors
Proteins are basic constituents of a cell. Some proteins such as molecular motors can transport cargo by walking on specific tracks. In molecular motors one often finds a sub-structure with distinct mechanical properties called coiled coil (shown in red) that has to resist unzipping forces produced by the motor. [read more]

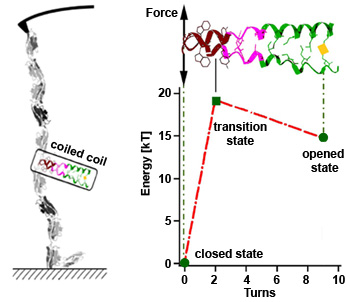
High coiled coil stability assures proper motor function
Using atomic force microscopy, we can stretch a single coiled coil structure taken from the molecular motor kinesin by anchoring it between the cantilever tip and the surface. The forces needed to open the coiled coil are higher than those produced by the motor, thus the structure stays mostly closed while the motor walks along a microtubule track. The related energy landscape shows that the coiled coil only opens completely, after the first 2 alpha helical turns are unzipped (transition state) which is energetically very demanding. This very high, local stability assures structural integrity of the motor and allows mechanical communication between the two heads which assures proper motor function. [read more]
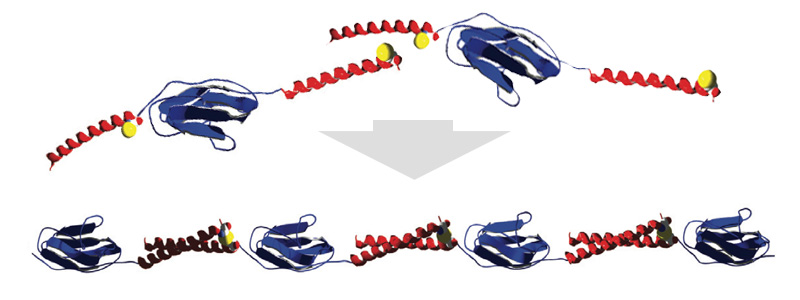
Coiled coils as building blocks for supramolecular protein assemblies
Coiled coils consist of two or more alpha helices specifically sticking to each other. Thus coiled coils allow to construct protein superstructures with designed mechanical properties. As a proof of concept we designed long, two dimensional chains of globular proteins (blue) connected via coiled coils (red). [read more]
The stability of knotted proteins
Proteins consist of a single polypeptide chain. In solution this long molecule folds into a three-dimensional structure, which is needed for the proteins biological task. Since it is topographically very unlikely, proteins usually don't show knots in their folds. For the few proteins that do show a knotted fold it was speculated that knots stabilize the protein, protecting it against unfolding. However mechanical unfolding of phytochrome needs forces that are typical for globular proteins, arguing against a stabilizing role of knotted folds. [read more]